Russ Colson
Minnesota State University Moorhead
Carbon Dioxide
is introduced into the atmosphere by many means, including burning of fossil
fuels. This introduction of excess carbon dioxide into our atmosphere has
the potential of effecting our climate by "greenhouse" warming. However,
there are also many processes that remove carbon dioxide from the atmosphere.
Like other problems in chemical differentiation (discussed in a previous
section of this course), the element carbon is not created or destroyed
in these chemical processes, but rather it is partitioned into different
phases. The chart below illustrates in a schematic way some of the
processes involved, and the reservoirs that carbon is stored in on Earth.
The percentages of carbon in each reservoir is shown (upper number in parentheses),
as well as the percentage of human-released carbon that is thought to be
in each reservoir (lower number in brackets).
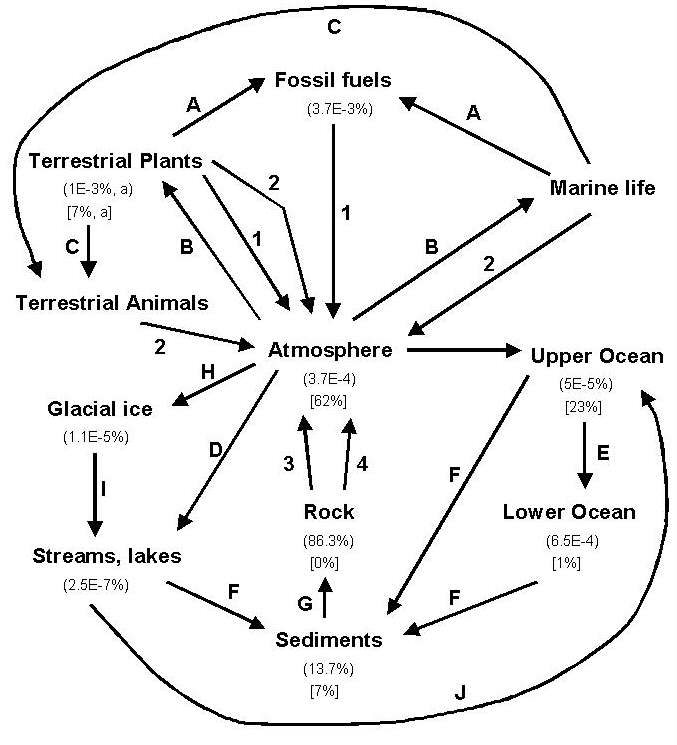
1: burning of carbon or carbon-containing molecules: C + O2 = CO2.
2: respiration, the oxidation of carbon by living things to generate energy
3: weathering of rocks that contain carbon to release CO2
4: heating of limestone to produce Calcium for use in cement. This releases CO2.
A: burial of living things in sediment and conversion of living carbon to coal or oil.
B: Photosynthesis takes carbon from the atmosphere and fixes it in sugars, cellulose, etc
C: Animals eat plants
D: CO2 partitions between water and air.
E: CO2 in water mixes between the upper and lower parts of the ocean.
F: living things and inorganic processes extract CO2 from water as sediment.
G: sediments are lithified, becoming rock.
H: CO2 partitions between the air and ice (as snow falls, for example)
I: Ice containing CO2 melts
J: rivers flow into oceans.
footnote: (a), the carbon in terrestrial plants includes carbon present
in animals.

1: burning of carbon or carbon-containing molecules: C + O2 = CO2.
2: respiration, the oxidation of carbon by living things to generate energy
3: weathering of rocks that contain carbon to release CO2
4: heating of limestone to produce Calcium for use in cement. This releases CO2.
A: burial of living things in sediment and conversion of living carbon to coal or oil.
B: Photosynthesis takes carbon from the atmosphere and fixes it in sugars, cellulose, etc
C: Animals eat plants
D: CO2 partitions between water and air.
E: CO2 in water mixes between the upper and lower parts of the ocean.
F: living things and inorganic processes extract CO2 from water as sediment.
G: sediments are lithified, becoming rock.
H: CO2 partitions between the air and ice (as snow falls, for example)
I: Ice containing CO2 melts
J: rivers flow into oceans.
Home Page (est.htm) Previous Page (est4b.htm)