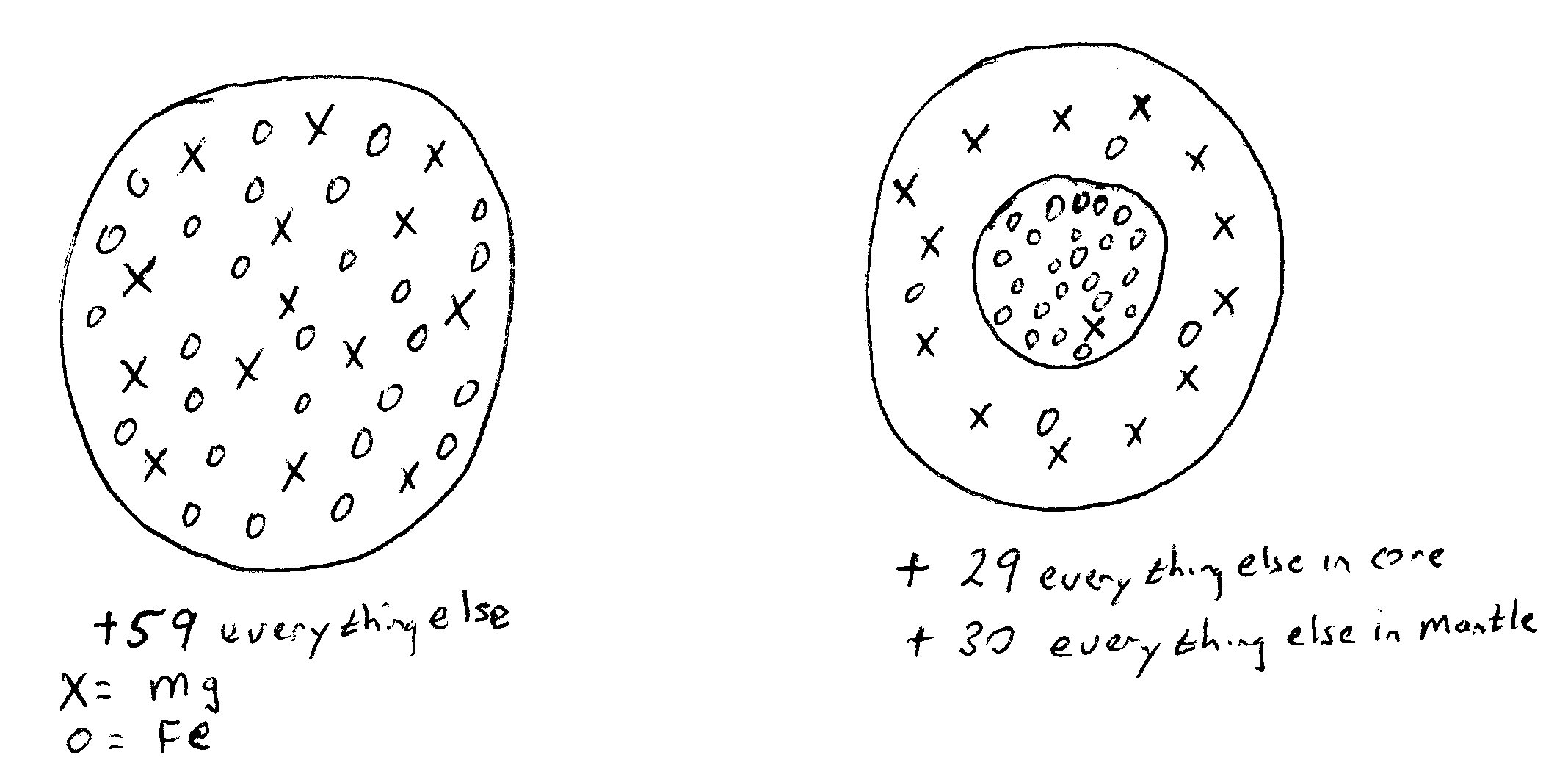
Answers to thought Puzzle: Core/mantle differentiation.
Suppose that initially the concentration of Fe (iron) in the Earth was
about 27%. The concentration of Mg (magnesium) was about 14%.
From laboratory experiments you measure the partition coefficient for Fe
in metal/Fe in silicate to be >>1. You measure the partition coefficient
for Mg in metal/Mg in silicate <<1. Which four of the following
do you expect to be true of Earth?
Mg in mantle = 14%, Mg in core = 14%
Fe in mantle = 27% Fe in core
= 27%
Mg in mantle <14% Mg in
core < 14% Fe in mantle <
27% Fe in core < 27%
Mg in mantle >14% Mg in core
> 14% Fe in mantle > 27%
Fe in core > 27%
The total amount of Fe and Mg do not change. However, the partition
coefficient shows that there will be a much higher concentration of Mg
in the mantle than the core (D<1) and higher concentration of Fe in
the core than the mantle (D>1). Since the total amount can't change
(we don't create or destroy atoms in this process) the mantle must have
Mg>14% and Fe<27% and the core must have Mg<14% and Fe>27%.
This can be shown as below.
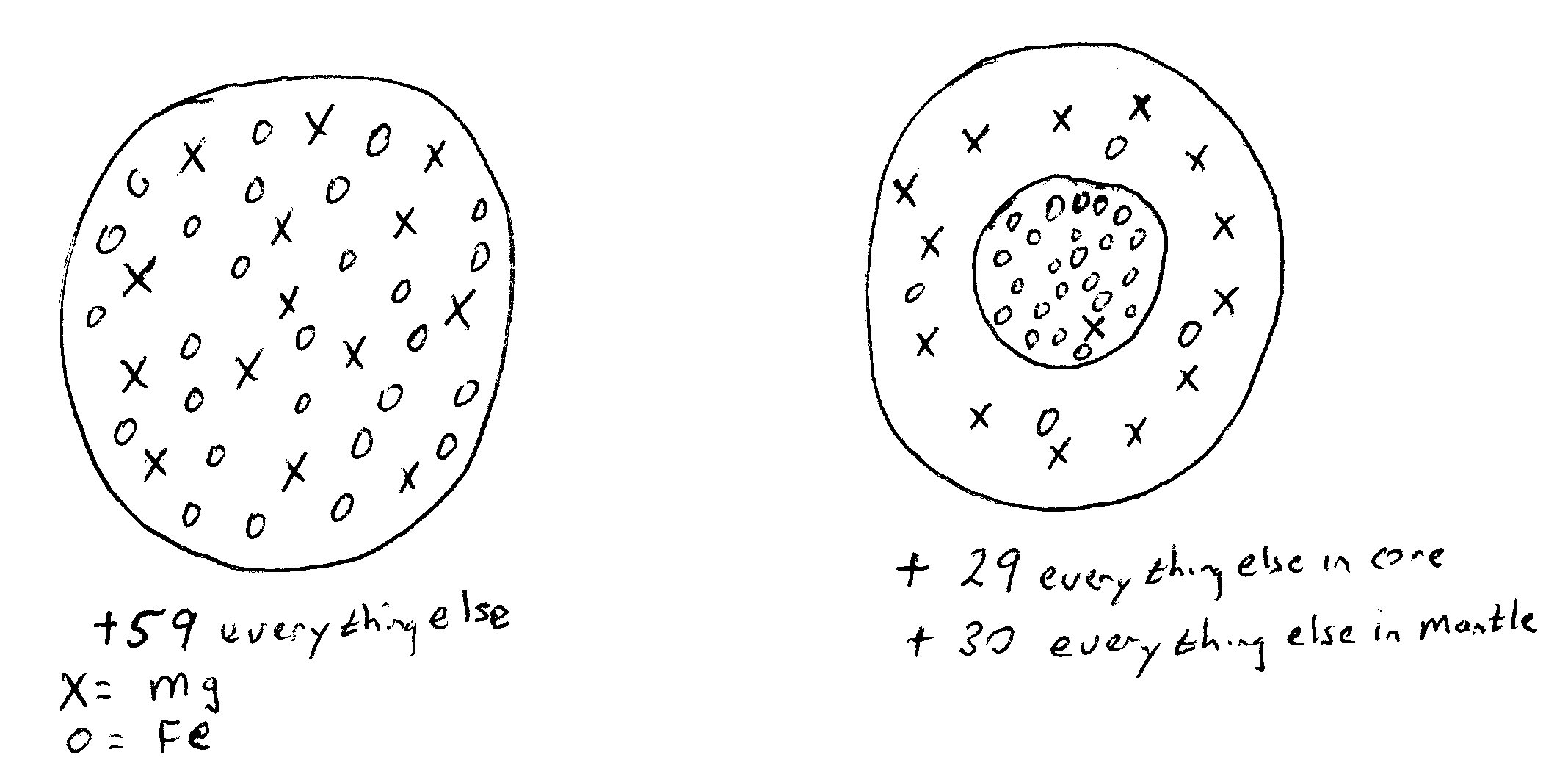
There are 100 total (14 Mg, 27 Fe, 59 Everything else)
There are the same totals as before.
14/100 = 14% Mg
But there are 23 Fe in core, 1 Mg
27/100 = 27% Fe
and 29 everything else = 53
59/100 = 59% everything else
1/53 = 1.9% Mg, 23/53=43% Fe
In the mantle, there are 4Fe, 13Mg
and 30 everything else = 47.
13/47 = 28%Mg, 4/47 = 8.5% Fe
Home Page (est.htm) Previous Page (est1b.html)