The baking soda and vinegar volcano is, for many young students, a memorable demonstration of how expanding gases can drive lava from a volcano. Here is an inquiry activity to expand that demonstration into an experiment that encourages a student to figure out how a volcanic eruption works. How is a baking soda volcano like a real volcano and where does a real volcano get its oomph?
To see how lava works, and where it gets its oomph, purchase some lava simulant. Simulant is a thing that simulates, or acts like, something else. You might find some lava simulant in the grocery store in the carbonated beverage section.
Cool your carbonated lava simulant in the refrigerator. Then, very slowly, draw some of the carbonated beverage into a large needle-less syringe (maybe a 60cc syringe or larger). Fill the syringe about a third full. Hold the syringe upright and expel all the air in the syringe so that only lava simulant remains. Put your finger over the opening to the syringe, shutting off all air inlet or outlet. Wrap your hand around the syringe and shake. Be sure not to let any air in.
Whoa! Where did all the "air" come from? Like gases from lava in a volcano, the gases in the syringe bubbled out of the carbonated water. Notice that the total volume of liquid plus gases inside your syringe has increased.
Try it again, this time filling the syringe 80-90% full. Again shake vigorously. This time the gas isnít as free to expand into the syringe because the syringe plunger "pegs out" at the end of the syringe. Pressure builds! With any luck, you can have a small explosive eruption and make a terrible mess with sticky soda! If that doesnít work, tip the syringe sideways and take your finger off the opening. Voila! Instant eruption!
The carbon dioxide in the soda is really very much like the carbon dioxide and water gases naturally dissolved in magma. As pressure on the magma decreases during its rise through the Earthís crust, gases bubble out of the magma. The expanding gases expel the magma out onto the Earthís surface as lava.
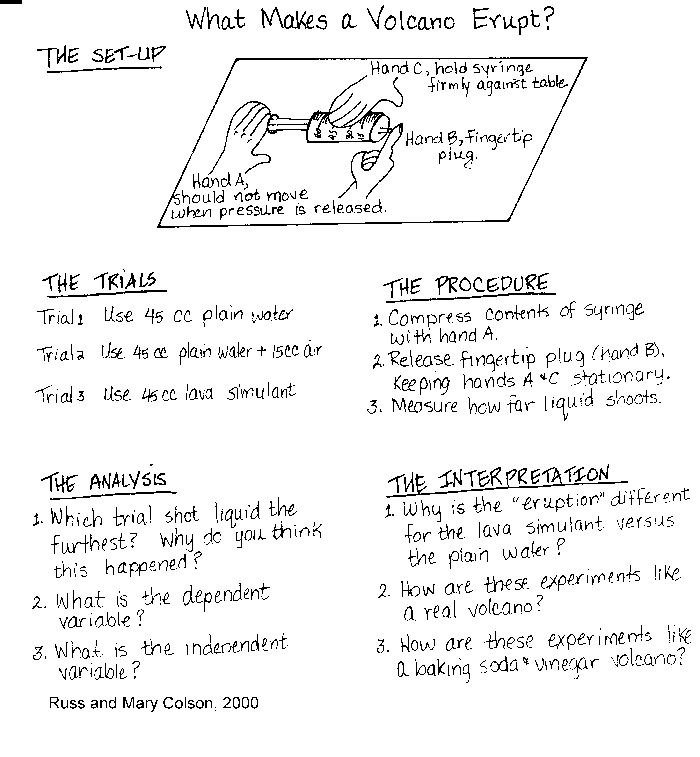
This activity can be extended to a variety of ages (an example activity
for Junior High students is shown in the figure below). It encompasses
four basic components of teaching Earth Science: 1) It is intriguing for
younger students because of the mystifying way in which the gases seem
to appear from nowhere. 2) It provides opportunity to practice experimental
techniques. 3) It provides the opportunity to investigate how changing
experimental conditions alters experimental results (and, for older students,
to consider dependent and independent variables). 4) Most importantly for
Earth Science, it provides the opportunity to practice the intuitive leap
connecting qualitative or quantitative experimental results to application
in the natural Earth.
Figure: An example best practice lab activity appropriate
for students in grades 7-12.

Home Page (est.htm) Previous Page (est1a.html)