
Planetary Geochemical Model Challenges
Earth Science Extras
by Russ Colson

The exercises in this lesson are intended to get you to think about how various geochemical observations, if made, would support (or not support) particular models for the formation of our solar system, Earth, and Moon. It is on the basis of reasoning such as this that scientists construct, and then test, various models for how our solar system evolved. To address the questions, think about how chemical properties would have changed if events proceeded as expected for the different models and what the chemistries would look like today.
Side note for science teachers: How to present evidence for a model is often taught less well in school than is simply describing or explaining a model. Connecting the dots from observational evidence, through reasoning, to model support is one of the hardest things for students to 'get.' Here is one simplified "follow the steps" approach to trying to teach 'arguing from evidence.' Often, we can think of evidence for a model as fitting into the following logical steps: If the model is correct then we would expect to observe <property 'X'>. We have have collected samples (or done experiments or made measurements) to check to see if property 'X' does indeed exist and have found that it <either does or does not>. Thus, this observation <either supports or undermines> the model.
After reviewing concepts of geochemical differerentiation, such as available at https://EarthSci4Teachers/ESE/gold_pollution_farmland-1.html and https://EarthSci4Teachers/ESE/gold_pollution_farmland-2.html consider the following challenges:
Before we can consider whether or not a planet has become differentiated (chemical elements re-organized into new packets with compositions different from the original state), we need to have some idea of what the starting original state was for our solar system. How do we know the composition of our solar system, and therefore what our total Earth composition is (or started out as) before differentiation?
One line of evidence suggesting that the material that makes up our solar system is very similar to the composition of our sun (which contains most of the mass of our solar system) is found by comparing the composition of un-differentiated chondritic meteorites with the composition of the Sun's photosphere (taking out the H and He that have been mostly lost from the meteorites).
One of the first, and arguably one of the biggest, events to be experienced by a planet is the differentiation of the planet to form a core. Perhaps during heating from bombarding meteorite impacts, a metallic phase (for terrestrial planets) separates from silicate phases and the greater density of the metallic phase causes it to migrate to the gravitational center of the planet, taking its parcel of elements with it and leaving the remaining silicate material to form a mantle depleted in those elements.
One model for the formation of the Moon that has been considered and tested for several decades is that the Moon formed early in our solar system history when a large planet-like body (perhaps chondritic in character) collided with the early Earth (the "Giant Impact" hypothesis). The idea is that this collision happened after the formation of Earth's core, but before any modern crust or other features of the modern Earth formed. The impact vaporized and melted a large part of both the Earth (mostly the mantle) and the impacting planet-like body, and some of this combined material was ejected into orbit around Earth where the more refractory components (less easily evaporated or melted) accumulated to form the Moon as the hot gas and ejected melt and dust condensed and accreted under gravity. Some of the combined material remained on the Earth or fell back to Earth after the impact. Some of the more volatile (easily evaporated) components, like Na, K, and water, may have escaped the Earth-Moon system entirely.
The model for formation of Earth's crust, in basic, simple form, is the following:
1) Mantle material partially melts (forming a combination of magma plus residual crystalline material).
2) The molten material, being less dense, rises and erupts to earth's surface (both volcanically and plutonically), while the residual material remains behind in the mantle.
3) The molten material freezes, making new crust
The relevant partition coefficients are therefore partition coefficients for elements between silicate minerals (the solid part) and silicate melt.
The mineral/melt partition coefficients for the elements Si, Al, and U are less than one. The mineral/melt partition coefficients for the elements Mg and Ni are greater than one. The relative masses of these elements, from greatest to least is: U, Ni, Si, Al, Mg.
The exact process for the formation of continents on Earth is difficult to infer because of the great age of these events (often older than the oldest rocks and obscured by ages of erosion of the rocks that might have told the story had they not been eroded away) and because these events were affected by tectonic process and partial melting/freezing processes that may have differed from modern processes in unknown ways. The problems below do not attempt to summarize key understandings of the evolution of continents on Earth, but ratherprovide examples of the kinds of logic that can give insight into these ancient events.
The "lunar magma ocean model" was proposed soon after the return of the first Apollo samples from the Moon. In simple concept, the idea of the model is that, after the condensation, accretion, and cooling of the Moon, the upper part, perhaps the upper few hundred kilometers, was reheated and melted by a period of higher-incidence meteorite impacts. As this 'magma ocean' cooled and froze again, more dense minerals crystallizing from the magma (perhaps olivine and pyroxene) sank to the bottom of the magma ocean and formed what would become the Moon's interior "mantle." Less dense minerals, like anorthositic plagioclase (a feldspar), floated upward to form 'rockbergs' which came together to comprise a significant part of the crust of the Moon. The residual melt, highly enriched in incompatible elements after removal of olivine, pyroxene, and plagioclase from it, was left behind at middle depths beneath the crust and above the settled olivine and pyroxene. This material came to be referred to as "KREEP", for the incompatible elements K (potassium), REE (rare earth elements), and P (phosphorous).
A signficant part of the evidence for this lunar magma ocean came from chemical patterns seen in the rare earth elements (REEs). The REEs all behave in a similar fashion because of similar cationic size and charge (typically +3), with slight differences in partitioning resulting from decreasing cation size going from the light REE's like Cerium (Ce), to heavier REE like Yttberbium (Yb). Except, one REE, Europium (Eu), can be divalent under the low oxygen (reducing) conditions on the Moon. This means that Eu stands out as different in its behavior.
We can compare all of the REEs in a particular rock sample to each other by "normalizing to chondritic composition" by dividing the measured concentrations of REE by the concentration of those same REEs in chondritic meteorites. If the a sample were undifferentiated, all the REE would normalize to a value of "one,' making a horizontal line on a graph at a value of one. In most common differentiation events, the normalized REE might have a pattern of gently decreasing or increasing values toward the higher mass (smaller size) cations. However, when Eu is divalent, such as under conditions of low oxygen activity, it stands out as different from the the other REE, giving a characteristic "Eu anomally" to the rare earth pattern.
An example of a REE diagram roughly characteristic of the Lunar highlands crust is shown in the graph below, in which REEs are normalized to chondritic meteorites, showing an example of a Eu anomaly.
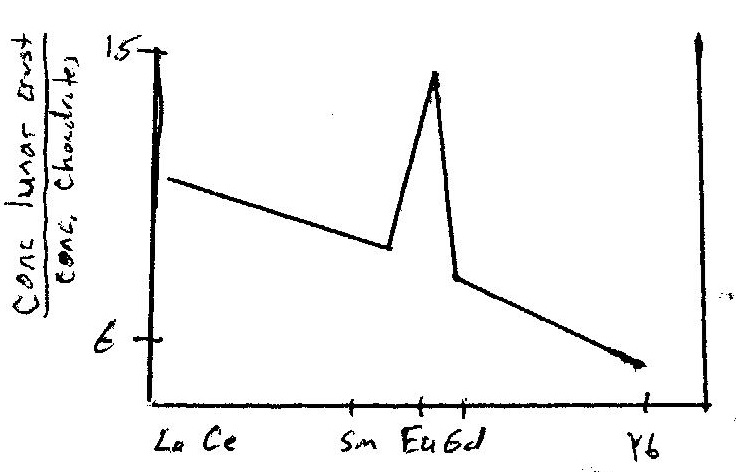
Notice that the concentrations of the REEs are all higher than chondritic (the normalized values are greater than one), consistent with the REEs on the Moon already being higher than chondritic values due to concentration in Earth's mantle when these elements were rejected from the metal core, prior to the giant impact that formed the Moon, a puzzle that we already examined above.
From the question above, we see that the Magma Ocean Model would predict that the Olivine and Pyroxene-rich lunar 'mantle' should be depleted in Eu relative to the other REE (a negative Eu anomaly). But how can we test this prediction? Humans have barely walked on the surface of the Moon, and we have not sampled the deep interior of the Moon (depths which would likely be out of reach even on Earth). So how can we figure out the Moon's interior composition?
Although we can't sample the Moon's deep interior, Apollo astronauts encountered volcanic deposits which are the partial melt that formed at those depths and then erupted to the surface, freezing into glass beads during eruption. We know the composition of those beads (brought back to Earth for analysis), and we can can experimentally measure the partition coefficients for the olivine and pyroxene that the Magma Ocean Model suggests should exist at depth, and so we have a means to estimate the composition of the solid olivine and pyroxene that is left behind in the Moon's interior. For the calculations below we are only going to consider pyroxene existing at depth in the Moon's interior.
The partition coefficients for pyroxene are the following:
Pyroxene/melt
The concentrations of these selected REE in volcanic green glass beads sampled by Apollo astronauts is the following (in parts per million = ppm):
Remembering that a partition coefficient equals the concentration in the crystal (pyroxene in this case) divided by the concentration in the melt, can you calculate the concentrations of Ce, Eu, and Yb that you expect to exist at depth in the Moon where the green glass volcanic beads originated?
To convert these values to a REE diagram, we need to divide these absolute concentrations by the concentrations in chondritic meteorites. Chondritic values might be roughly the following:
This yields the following normalized concentrations (no longer in ppm since the ppm in the Moon's interior and the ppm in the chondrites cancel):
last updated 4/5/2023. Text and pictures are the property of Russ Colson, except as noted.