Mineralogy-Petrology--Thin Sections Part 3--Carbonates
Earth Science Extras
by Russ Colson
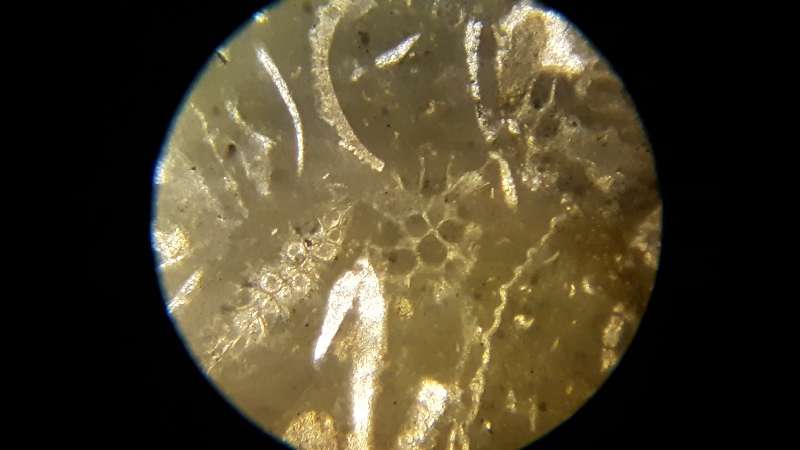
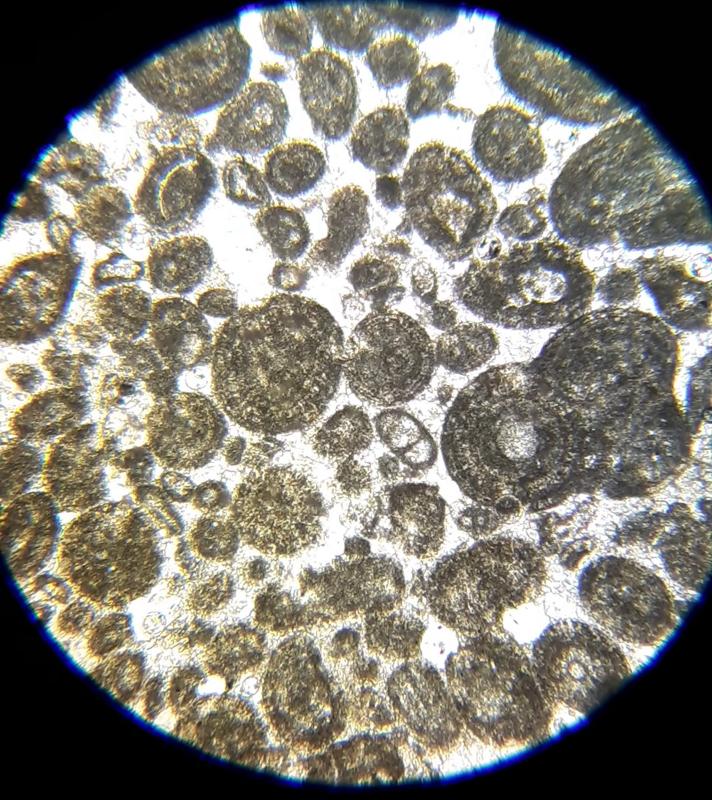
Photomicrograph of fossiliferous limestone with bryozoa, pelecypod shells, trilobite fragments, and brachiopod shells under plane light.
Introduction
Carbonates are sedimentary rocks composed largely of calcite, aragonite, and dolomite. Calcite can be identified in thin section by its rhombohedral cleavage and very high interference colors (often so high as to appear almost white, superficially similar to a low interference color but with little rainbow flashes of color here and there in the mineral).
We can classify carbonates acccording to two observable features seen in both hand sample and under the microscope. These two are
1) Grains (called allochems)
and
2) Matrix (called orthochem)
Types of Grains (allochems) include the following
Fossils: Fossils can include pelecypods, gastropods, brachiopods, ostracods, crinoids, bryozoans, coral, and so on Shape can be used to identify the fossil type in in hand sample--internal structures and characteristic shapes can help identify them under a microscope.
Ooids (or oolites): These are sand sized concentric, spherical polycrystalline carbonate grains. They typically form by repeated layering of a nucleus (e.g. qz, fossil) by chemical precipitation from marine or hypersaline water. The particles need to be agitated as they grow in order to achieve the concentric character, precipitating calcite equally on all sides. Consequently, these particles often form in a shallow environment with water movement, such as a wave-swept shoal. They can exhibit cross-bedding and other sandstone-like features.
Peloids: These are spherical or ellipsoid carbonate mud pellet with no internal structure. A common type of peloid is fecal pellets (which typically have a uniform size). Peloids can also form from internally-structured particles such as ooids through secondary processes--for example, certain boring algae can destroy internal structure of other types of particles, creating a peloid.
Limeclasts: These are fragments of lithified or partially lithified preexisting limestone. Intraclasts are a subset of limeclasts that originate penecontemporaneously from within the same depositional basin, although they may have been transported a short distance by a storm.
Types of matrix (orthochems) include the following
Micrite: This is clay- or silt-sized particulate calcium carbonate sediment. Origin of this fine-grained carbonate sediment might be algae which break apart on death, chemical precipitation directly from sea water, or some fish with tough teeth might chew on carbonate reef material and poop micrite! Micrite is usually deposited as aragonite that later converts to calcite (remember the stability fields of calcite and aragonite that we considered in our lesson of phase diagrams), although Mg concentration in sea water affects the likelihood of aragonite precipitation.
Spar: This is crystalline calcium carbonate crystals grown in voids in rock (sometimes including cavities within fossil shells). It can also be dissolved, remobilized, and recrystallized micrite. Origin is often often CaCO3 precipitated inorganically during diagenesis of the sediment.
Naming Carbonates
Carbonate rock names have two parts. A "first name" that identifies the dominant grain (allochem) in the rock, and and a "second name" that identifies key characteristics of the matrix (orthochem).
First names might include things like "oolitic", "peloidal", "fossiliferous", or "intraclastic", or more specific words like "brachiopod" or "crinoidal."
Second names fall into two categories.
1) The simplest, after Folk (Folk, R.L., 1959, Practical petrographic classification of limestones), is to simply give the name of the matrix material, either micrite or spar. Names of rocks using this system include (sometimes shortening the first name for convenience) oosparite, biopelmicrite, intrasparite, crinoidal micrite, and so forth.
2) A more involved system, after Dunham (Dunham, R. J., 1962, Classification of carbonate Rocks according to depositional texture), gives more information about the matrix. This classification hinges on how much of the rock is matrix and how much is grain. If the grains create a grid of touching particles, such that the rock would not collapse if the matrix were removed, we say that it is grain supported. Otherwise it is mud supported. We further divide the samples by how much mud and how much grain there is for a mud-supported sample.
So the "last names" by this classification include the following:
Mudstone (the sample is mud supported and there are less than 10% grains)
Wackestone (the sample is mud supported and there are more than 10% grains)
Packstone (the sample is grain supported and the matrix is micrite)
Grainstone (the sample is grain supported and the matrix is spar)
Boundstone (the rock was cemented (that is, bound) during deposition rather than by later cementation processes. This can, for example, happen with modern scleractinian corals which grow reefs both upwards and laterally, creating an interlocking rock grid that is 'bound' prior to diagenetic cementation.
Names using this system might include things like fossiliferous wackestone, oolitic grainstone, peloidal mudstone, intraclastic packstone, and so forth.
For the following exercises, we will use the 2nd of these classification schemes.
Practice naming carbonates:
In the following problems, grains of all types (fossils, ooids, peloids, and limeclasts) are symbolized by circles. The matrix is symbolized by one of the two patterns:
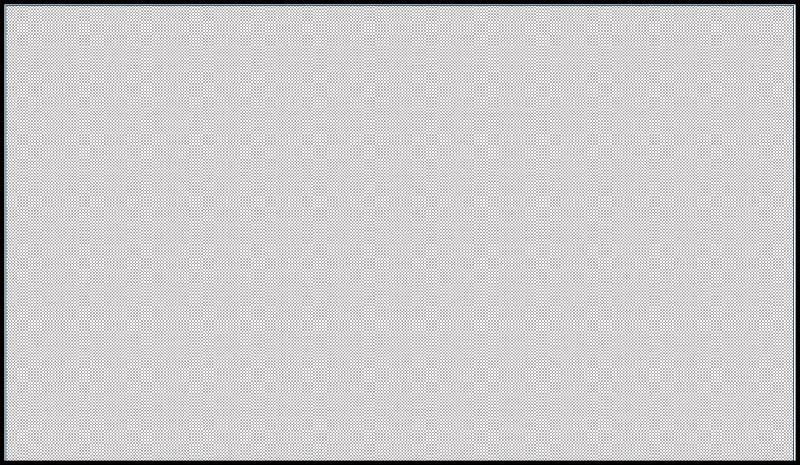
Symbolic Micrite is shown below:
Symbolic Spar is shown below
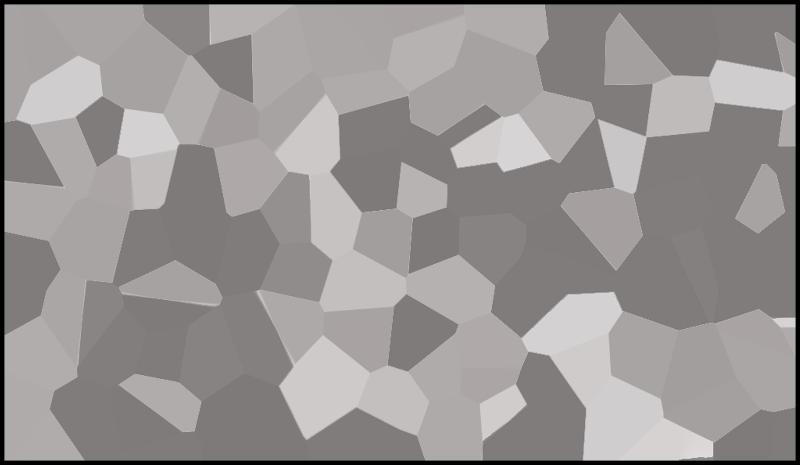
For each of the following, identify the second name for the Dunham classification.
Carbonate Thin Section Tutorial
In preparation for identifying grains (allochems) and matrix (orthochems) under the microscope, work through the tutorial below that looks at sevedral different regions of two different carbonate thin sections. Click on the image to begin the tutorial.
A short summary from the tutorial
Micrite: fine grained matrix, often brown or grey
Spar: crystalline matrix, usually calcite
Mollusks (e.g. bivalve): typically recrystallized from aragonite, with a mosaic of calcite crystals taking on the shape of the original shell
Brachiopod: typically not as extensively recrystallized as mollusks, and so you can see internal structures in the shell, such as a sub-parallel fabric
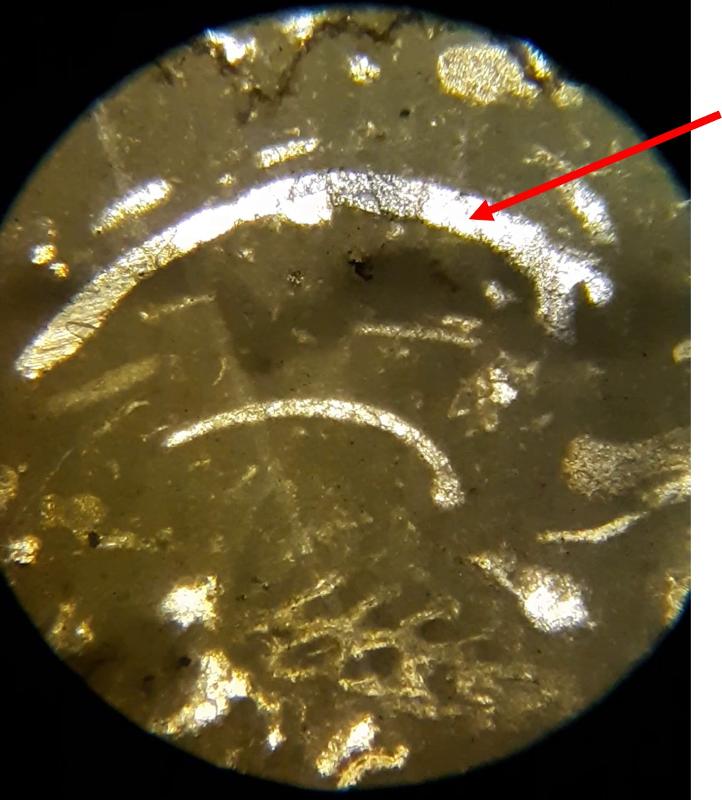
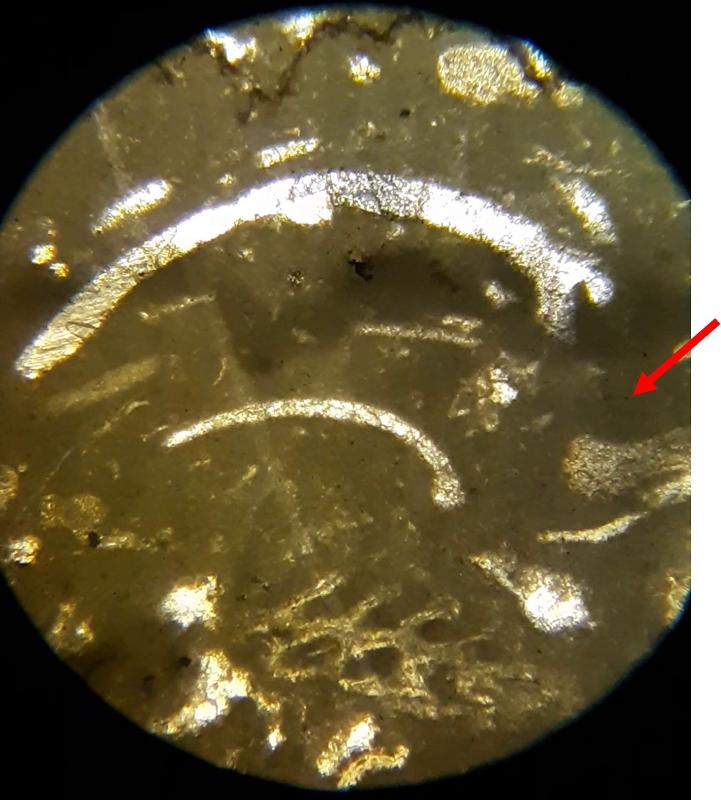
Trilobite: common sherherd's hook, and, when not recrytallized, an extinction pattern in which the zone of extinction sweeps from the center toward the ends of the shell
Bryozoa: cellular structure
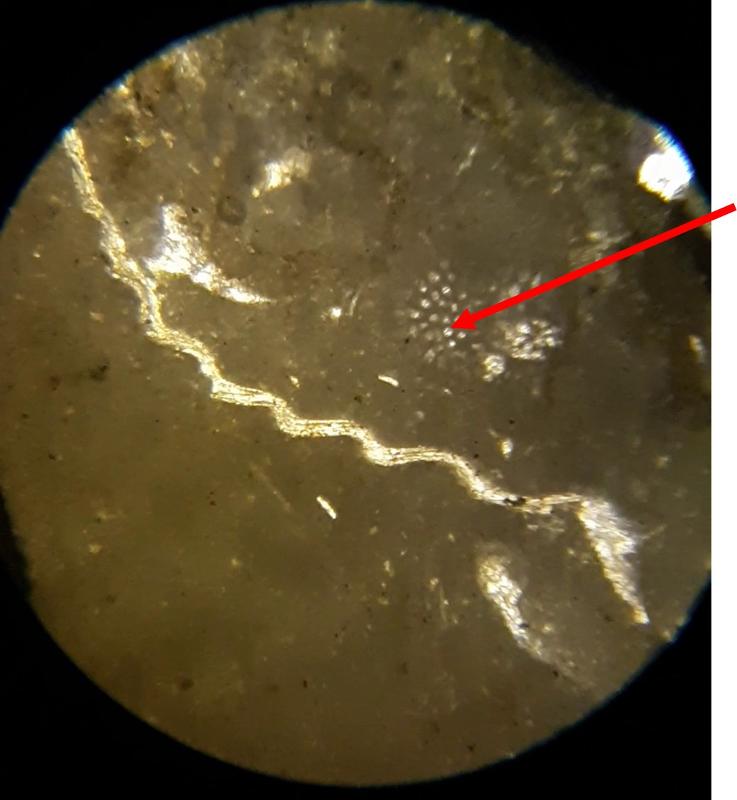
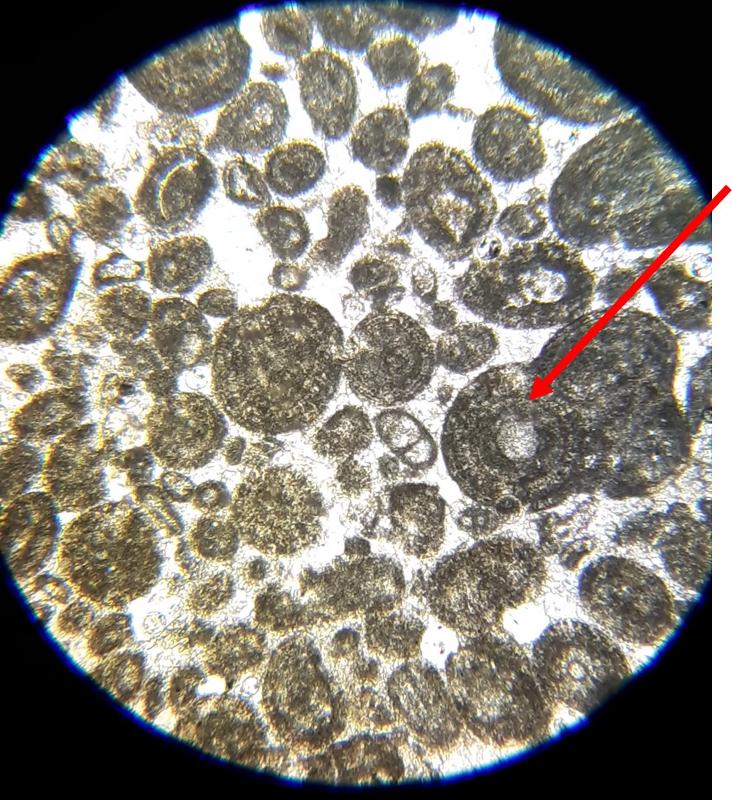
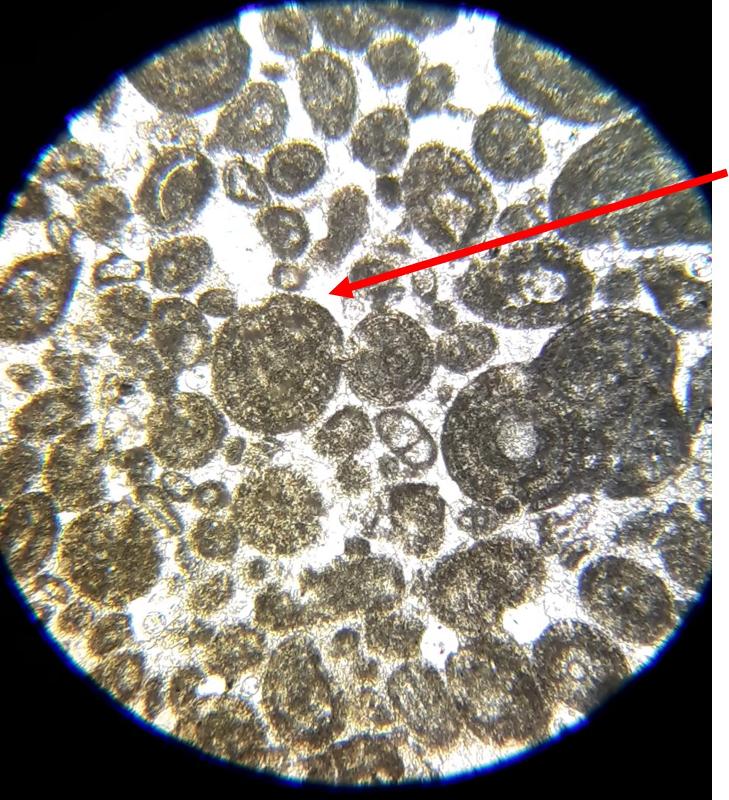
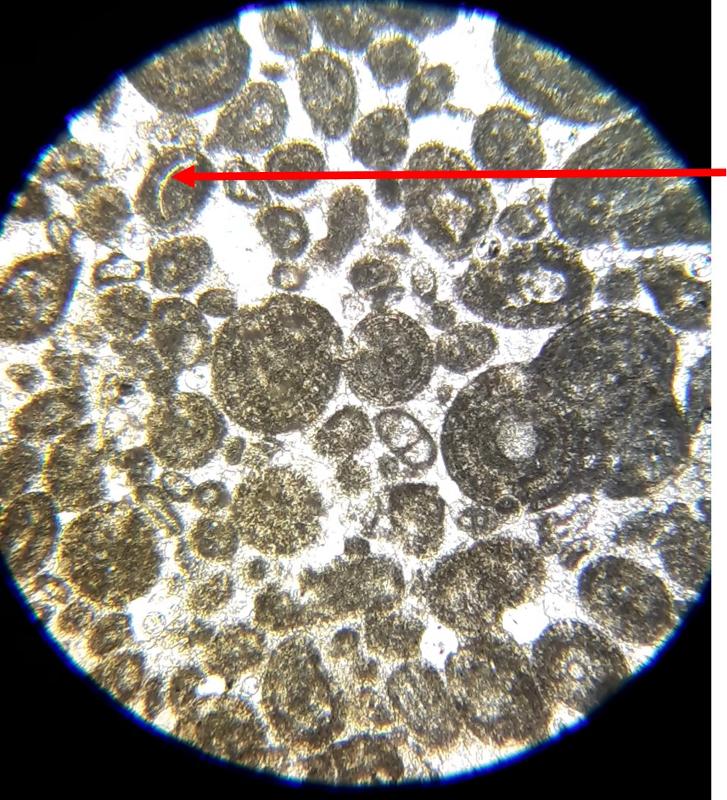
Oolite: spherical particles with a concentric structure in the interior
Pellet: evenly-sized spherical particles without interior structure
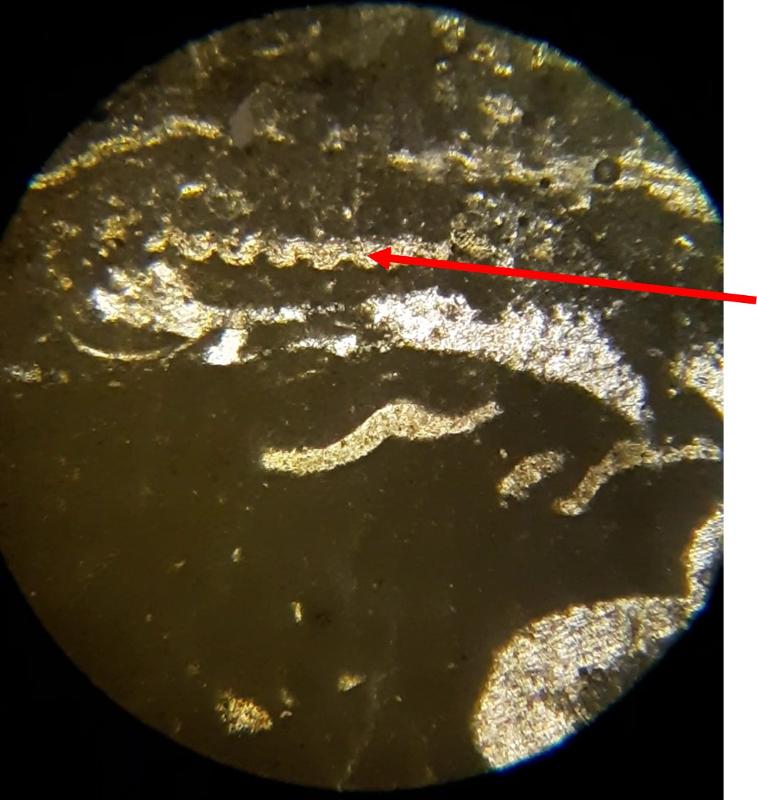
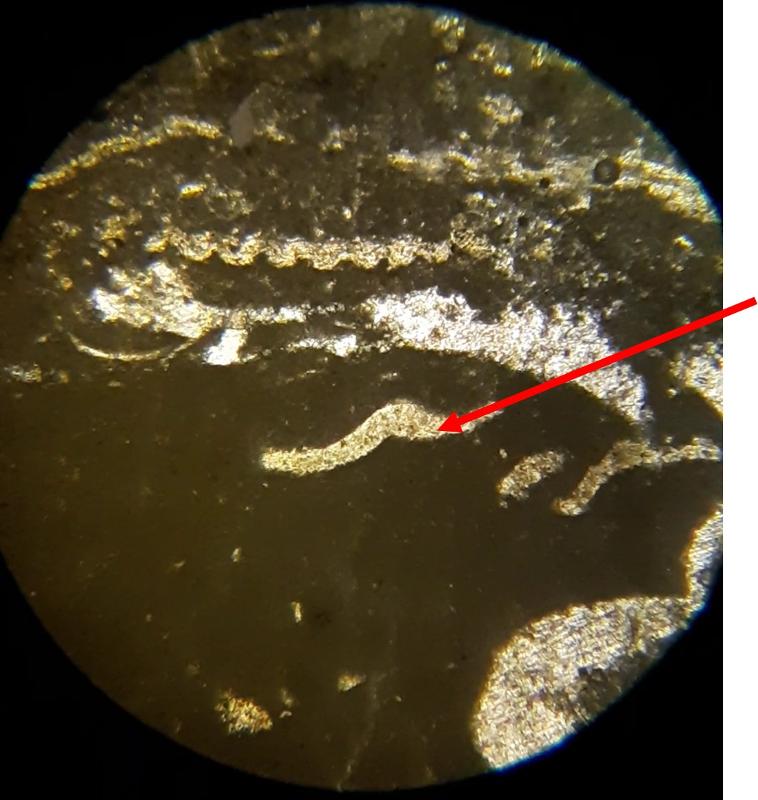
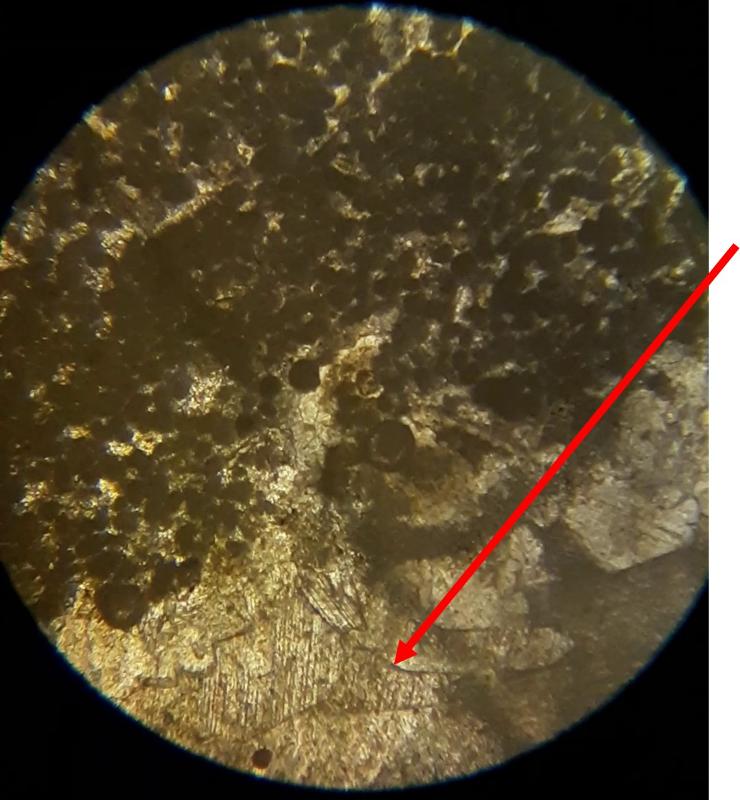
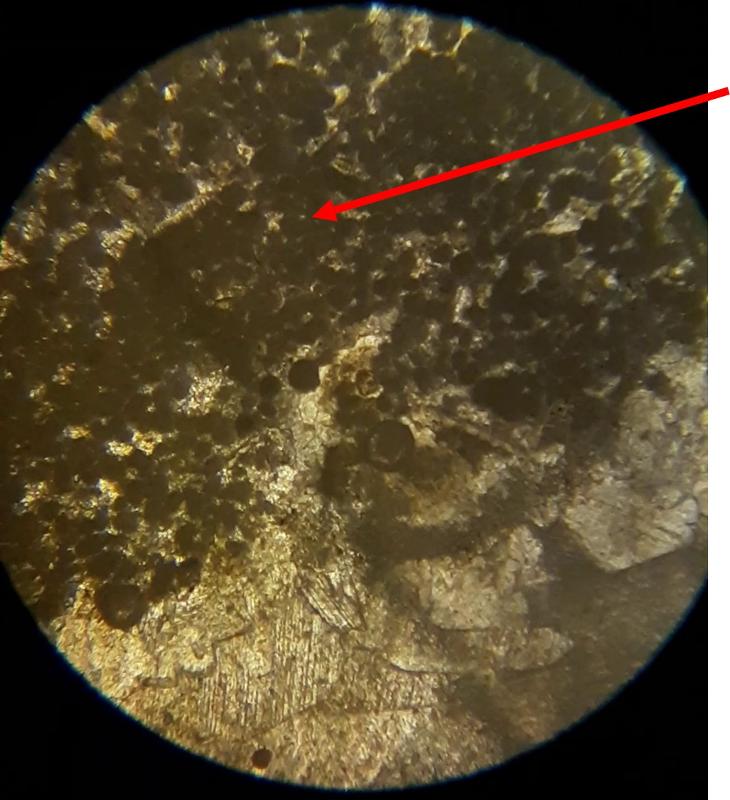
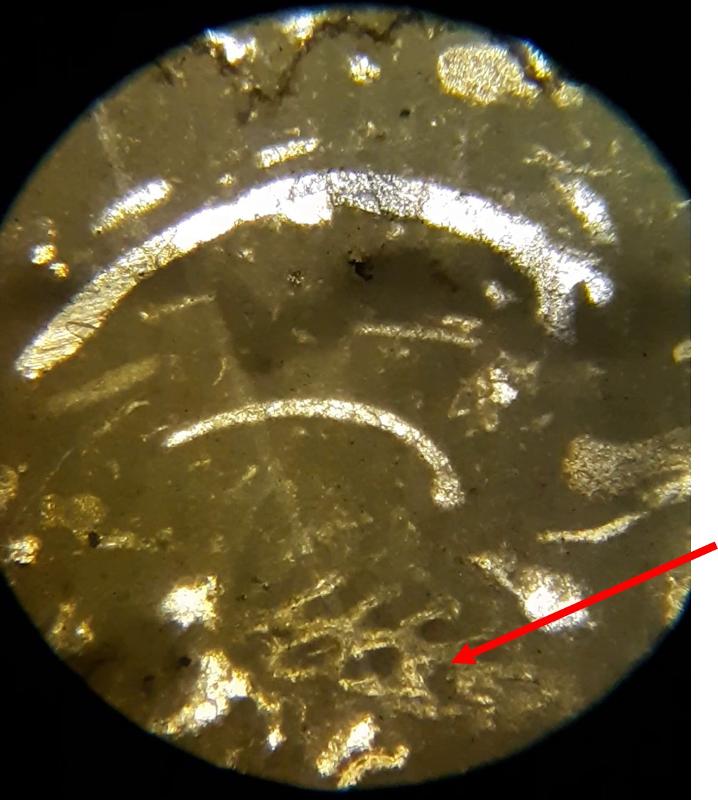
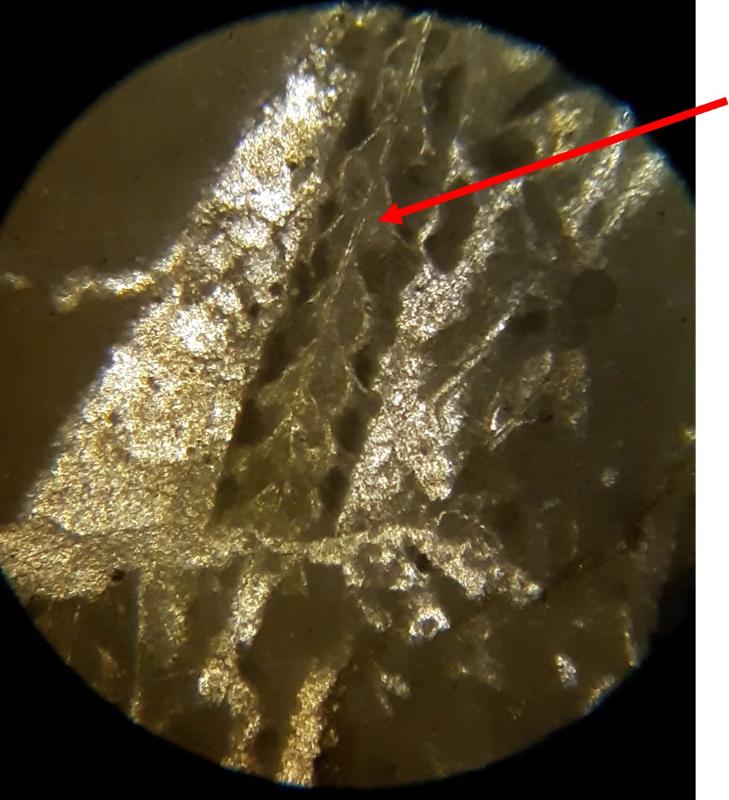
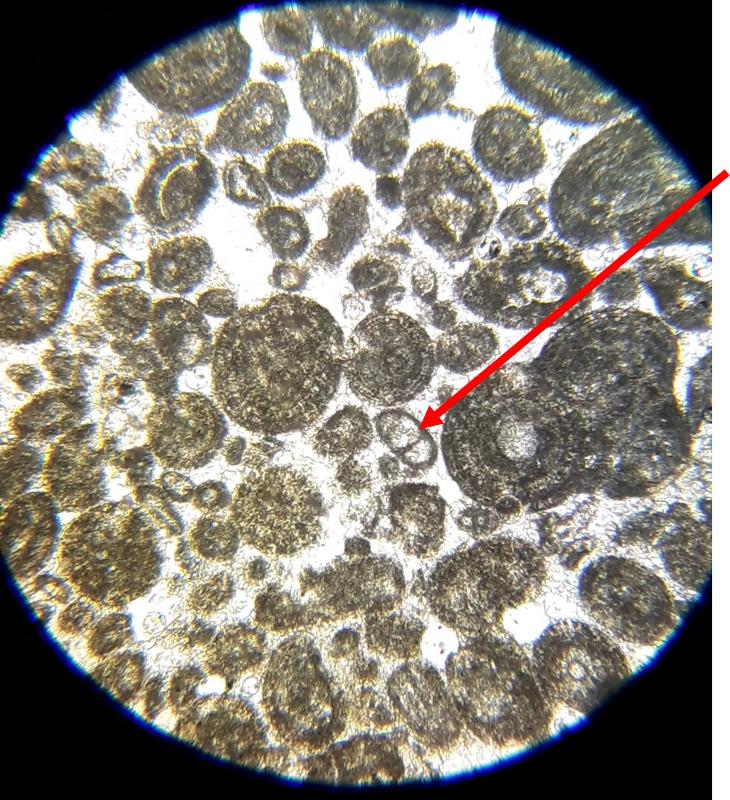
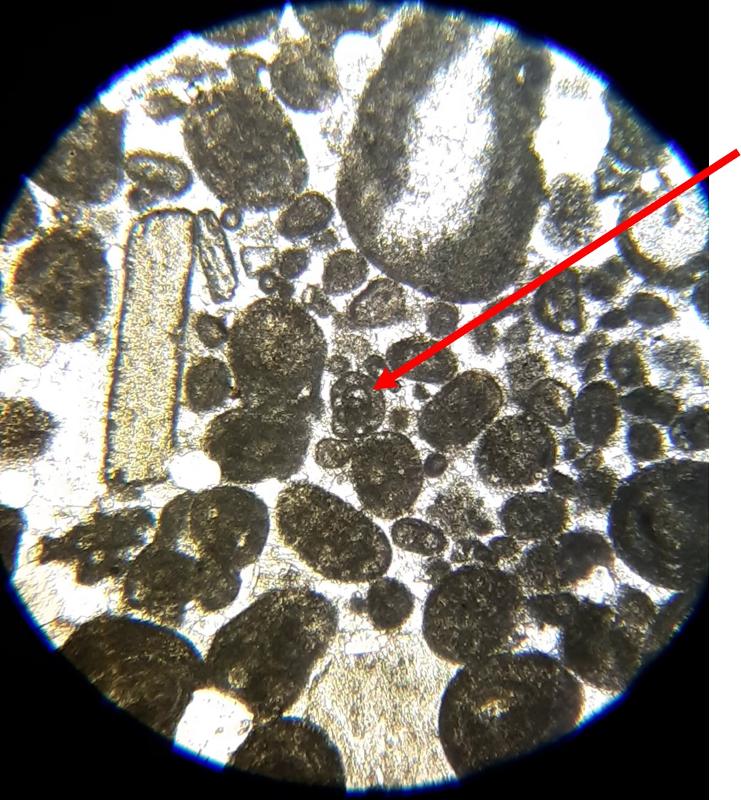
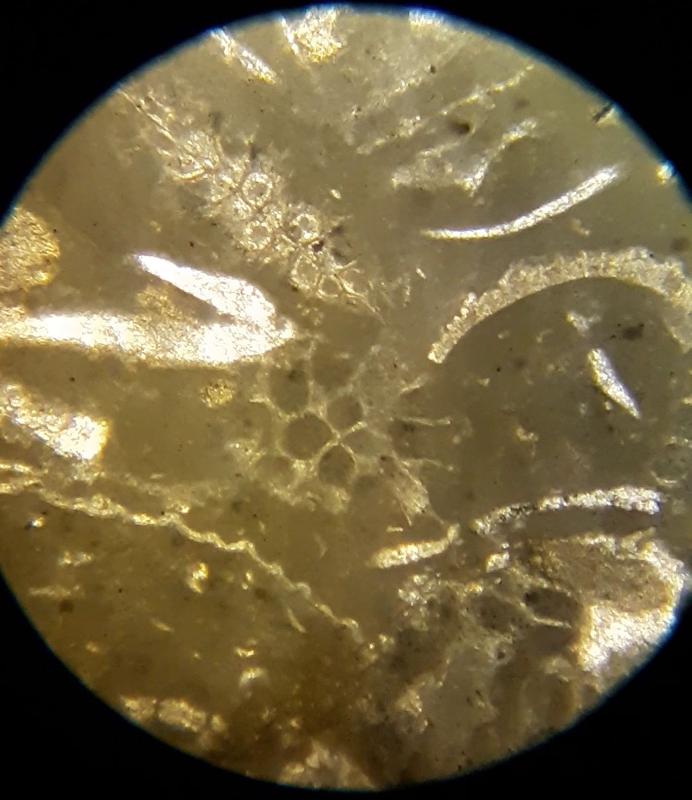
Take a look at the feature indicated below. What do you think this might be?
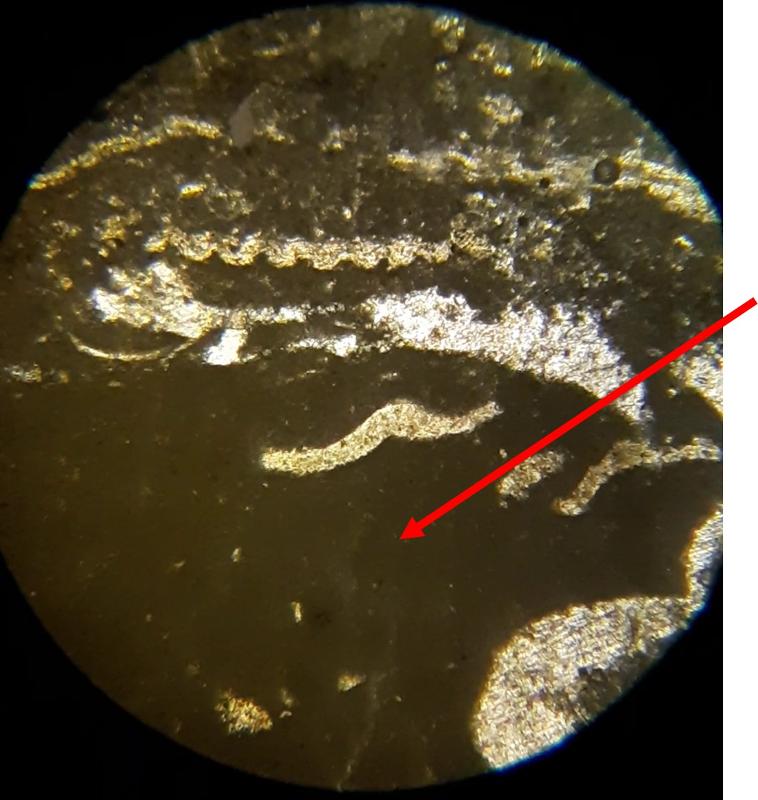
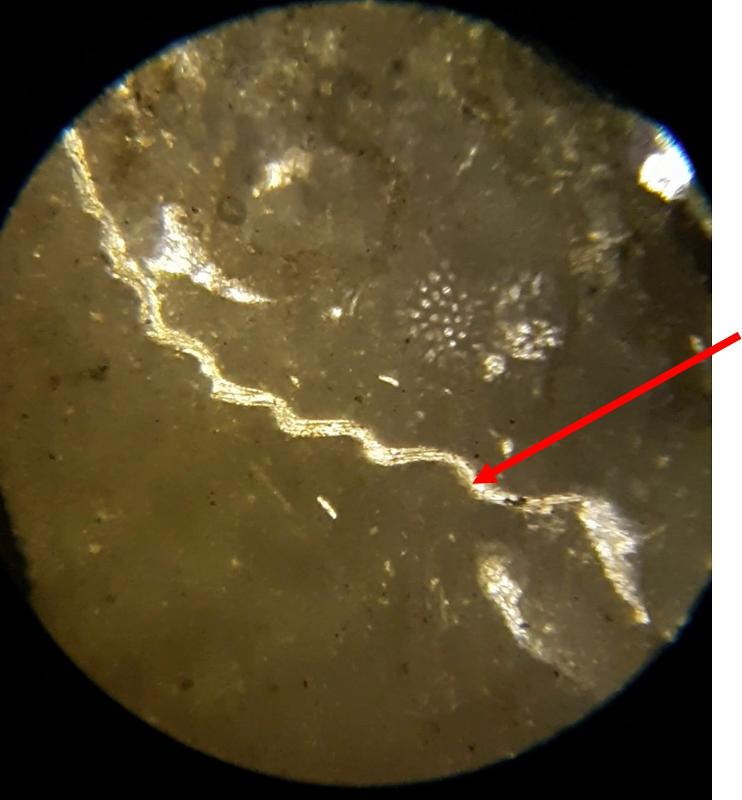
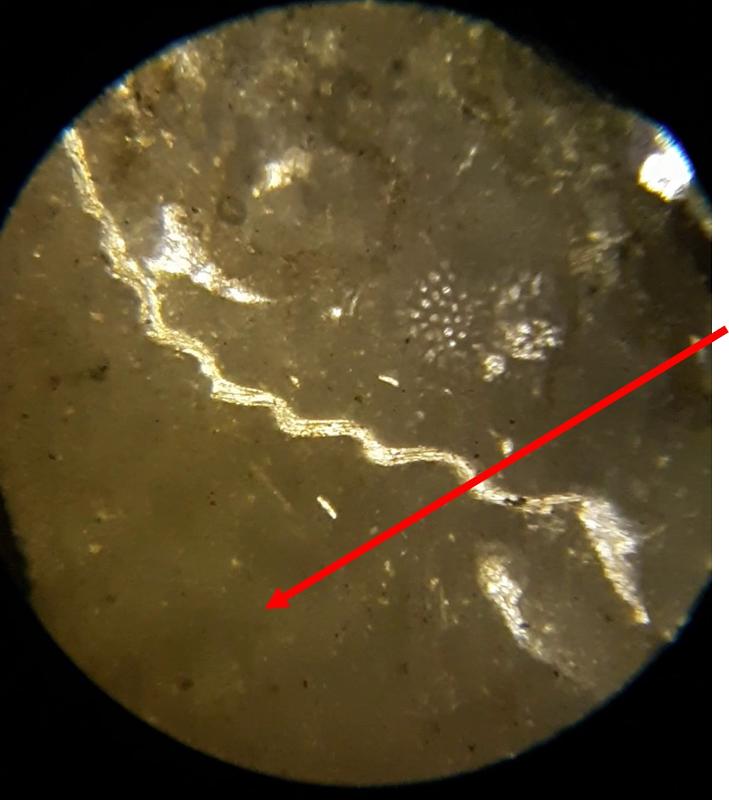
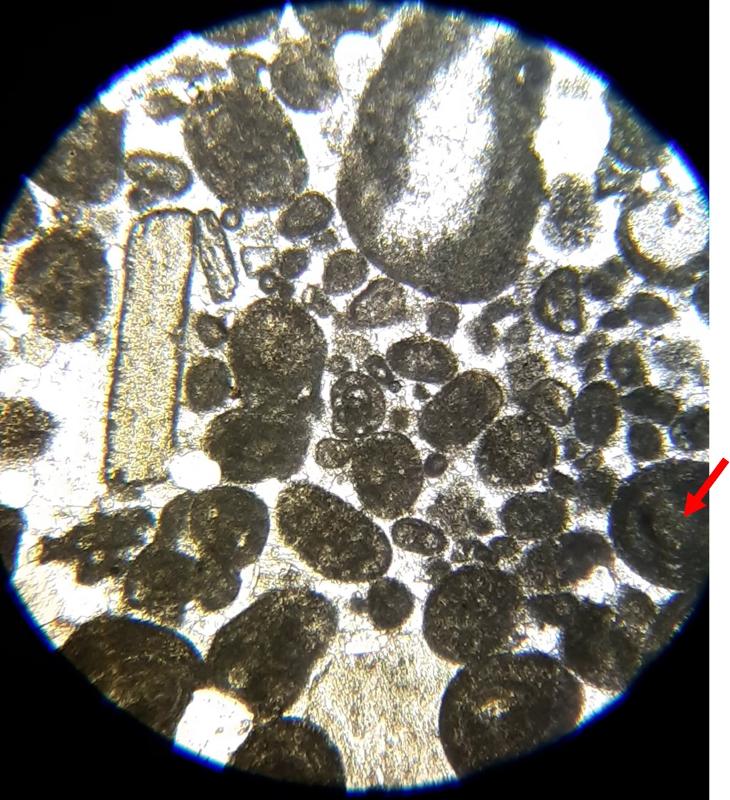
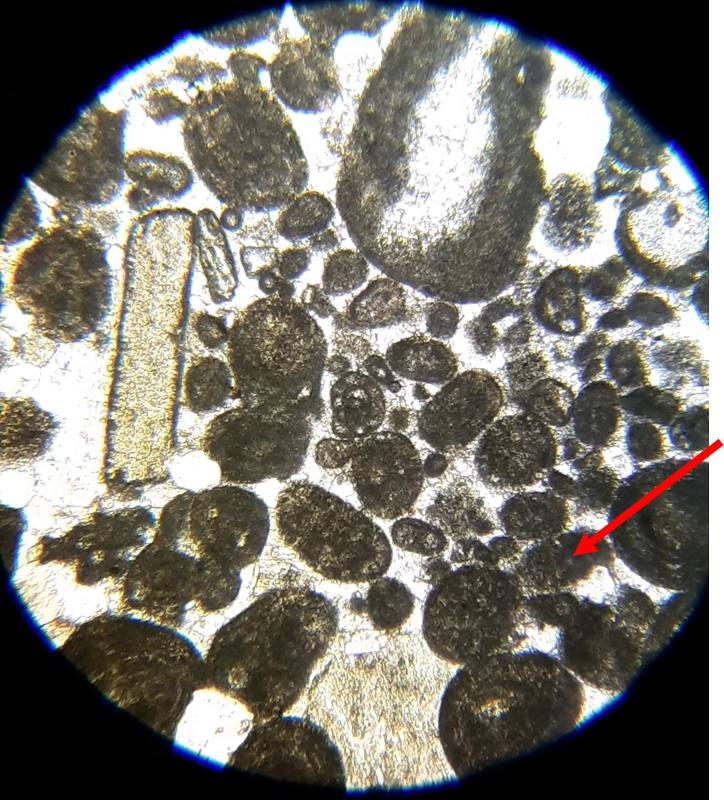
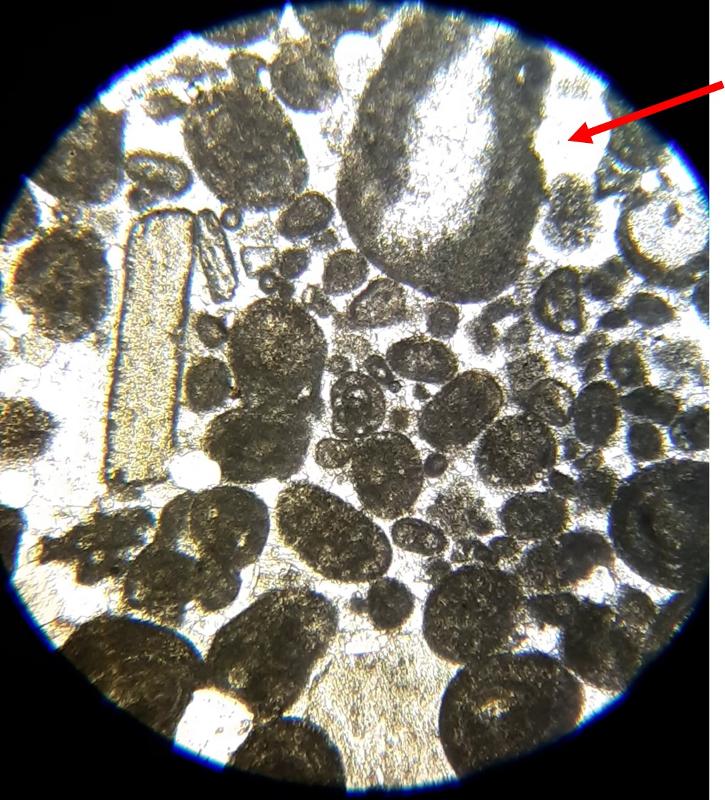
Below are a few additional features, including limeclasts, pore spaces, and a look at a small chambered grain perhaps similar to the one in the previous image.
Naming the two rocks we've looked at
Last consideration of what you are learning in a lab like this
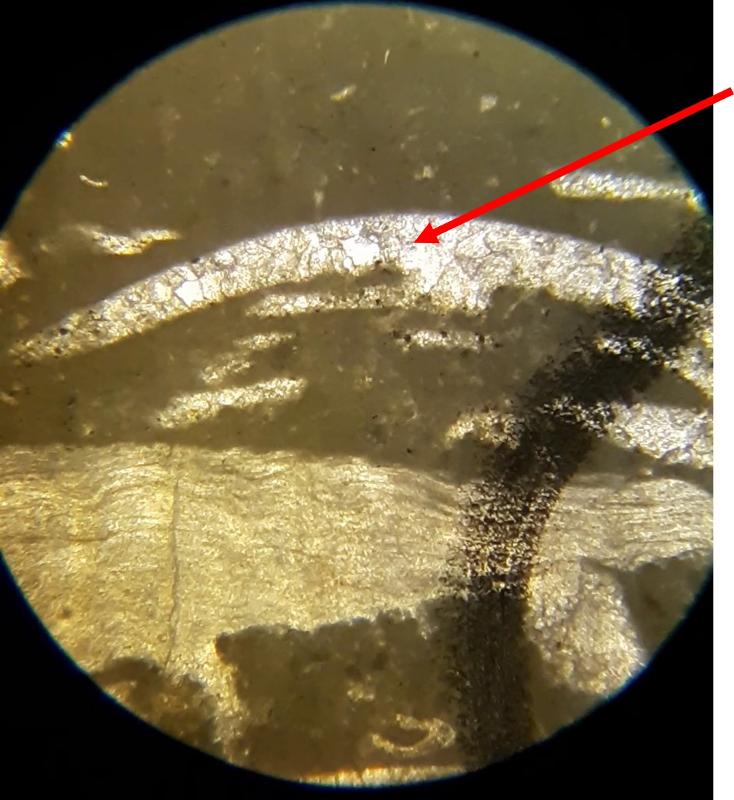
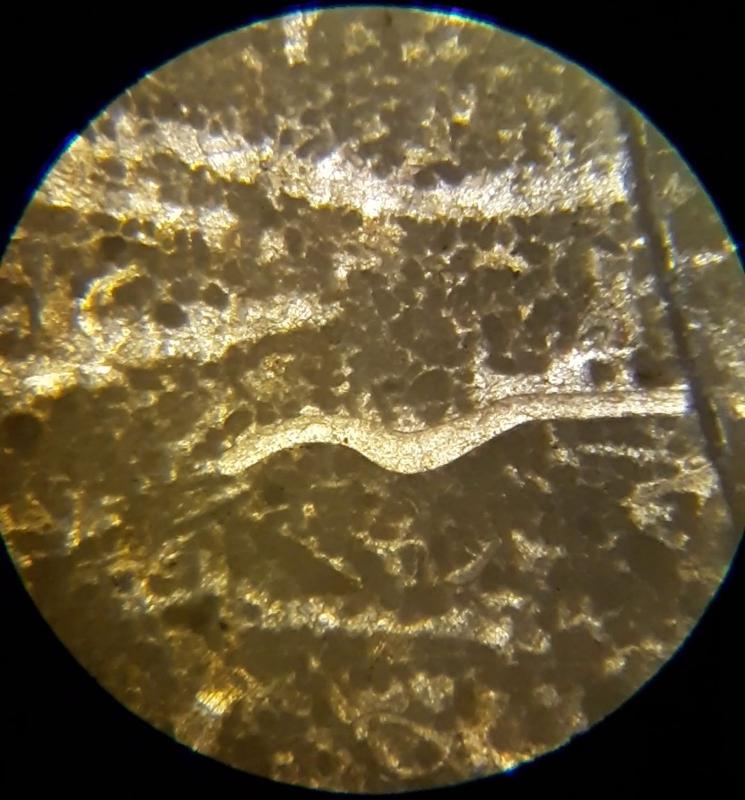
Thin section petrography is about more than 'knowing the features and looking for them'. It takes practice to recognize features, so this lab-like exercise is about practice making nuanced obervations. The importance of experience and practice are made even more clear when you consider that fossils can look quite different depending on the angle that the thin section slices through the feature, and can look even more different in samples with different species from different time periods--we have only looked at two carbonate samples. The most subtle feature in these exercises might be the 'sweeping extinction' of the trilobite fossils when viewed under crossed Nicols. Below is another opportunity to spot this feature, in case you have been struggling to see it. Click on the image to view in a separate window and rotate the stage.
Distinguishing Calcite from Dolomite
Another important feature of carbonates, particularly samples from the early Paleozoic like the fossiliferous wackestone we have looked at, is how much of the calcite has been converted to dolomite. Calcite (CaCO3) can be converted to dolomite (CaMg[CaCO3]2) during diagenesis of the rock (what happens after deposition and burial), and dolomitization is a common feature of carbonates, particularly older ones. Dolomite (and other carbonates that are less-soluble in acid) can be distinguished from calcite (and other carbonates that are more soluble in acid) by dipping the thin section in a solution of mildly acidic alizarin red-S solution. This solution will stain soluble minerals--like calcite--red, while less soluble minerals--like dolomite--are not stained. In the images below, you can see patterns of 'veins' of dolomitization, selective dolomitization in single fossil fragments, and a few diamond-shaped dolomite crystals in a calcite matrix. Make sure you can find all of these in the images below. Also, try to identify fossil fragments!
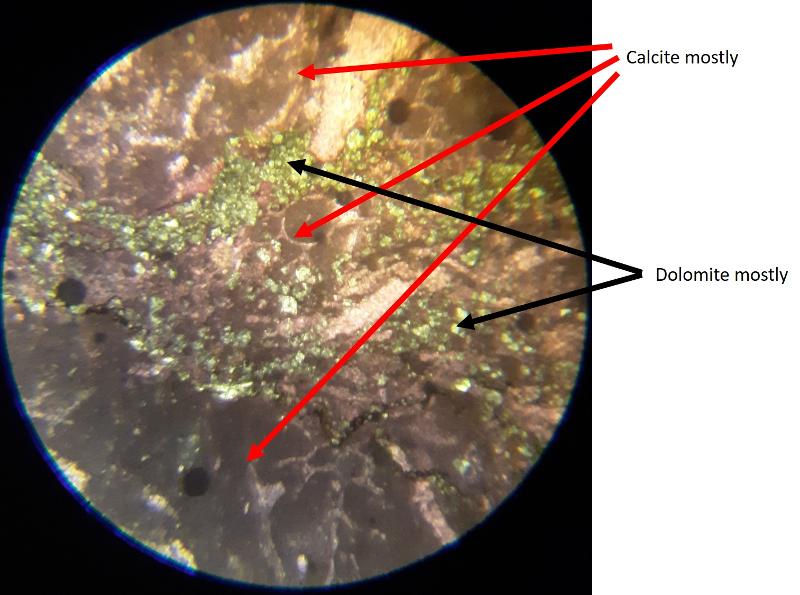
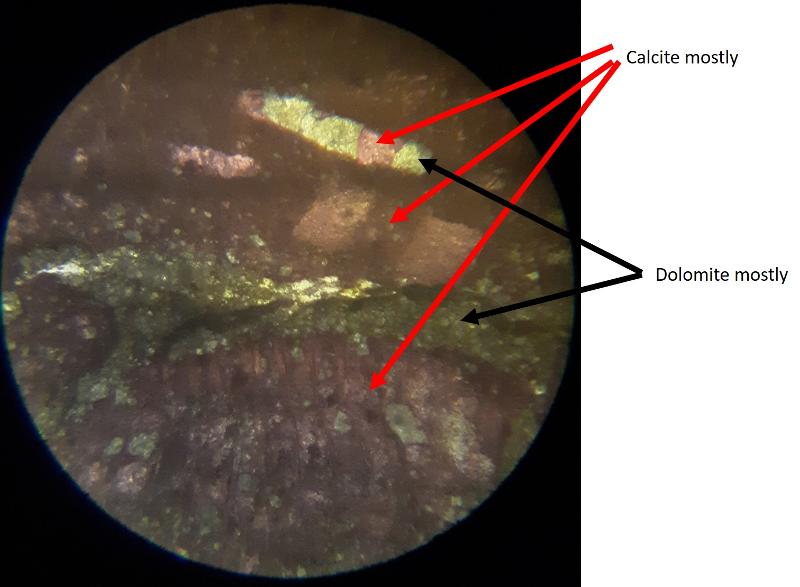
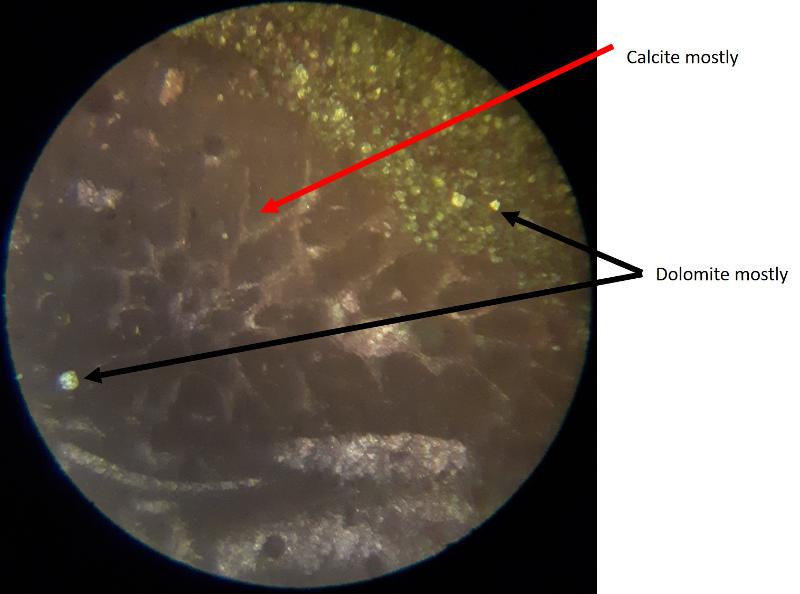
This ends Part 3--Carbonate grains and matrix.
last updated 4/24/2021. Text and pictures are the property of Russ Colson.