
Earth Science Extras
by Russ Colson

Mendenhall Glacier, Alaska 2002.
First, let's test your understanding of the lecture on atoms and isotopes.
.
.
.
If you missed any of these, you might want to go back and rewatch the lecture, taking notes and thinking about what the ideas mean.
You should also take notes as you work through the thought puzzles below. Remember, the purpose of taking notes is not to store information, but rather to spur your own thinking and to start the process of making ideas your own.
Since isotopes of a particular element have the same number of protons, their broad chemical properties are very similar. However, because they have different mass, they will behave differently in some situations. For example, water that contains a heavier isotope of oxygen will evaporate less easilty than water that contains lighter oxygen..
Studies that use stable isotopes will usually look at lighter stable isotopes. The reason for this is that there is a bigger proportional difference between, for example, 12C and 13C than between 206Pb and 207Pb. As another example, oxygen 18 differs from Oxygen 16 by 12.5%, whereas Uranium 235 differs from Uranium 238 by only 1.3%, Therefore, there will be less effect on lead (Pb) and Uranium (U) from the difference in mass of the atom. We often use isotopes of heavier radioactive elements to tell us about age, but that is another story.
Both temperature and biological activity can sometimes separate isotopes of different mass, giving us a way to infer past temperature (either for climate or for ore-forming fluids) or past metabolic activity (for example, C3 and C4 plants have different metablic pathways, as do legumes and non-legumes, resulting in different isotopic ratios).
Some Important Stable Isotopes in Geology:
16O. 17O, 18O (Atomic number oxygen = 8) We usually look at 16 and 18 because they have bigger difference in mass and so are affected more by (for example) evaporation processes.
12C, 13C (Atomic number carbon = 6) (note that 14C is radioactive)
14N, 15N (Atomic number carbon = 7) (can be used to distinguish between C3 and C4 plants in ancient diets)
32S, 33S, 34S, 36S (Atomic number sulfur = 16) We usually look at 32 and 34 because they are the most abundant (32=95%, 34=4.2%)
36Ar, 38Ar, 40Ar (Atomic number argon = 18) Can be used to identify the loss of Earth's primordial atmosphere (mainly Ar-38) from a later volcanic atmosphere enhanced in radiogenic Ar (40). "Radiogenic" means that that isotope has been derived by the radioactive decay of a precursor atom.
concept of delta values:
We compare diffferent isotopic ratios by comparing the stable isotope composition of our sample of interest with a standard. To do this, we use the concept of a delta value.

The units of a delta value are in parts per thousand (thus the multiplication by 1000) and the value is a ratio of ratios, with the proportion of an isotope in a sample of interest divided by the proportion of those same isotopes in a standard.
By convention, the heavier isotope is placed in the numerator of the ratios. For example, for the oxygen isotopes O18 and O16, the delta value is calculated by
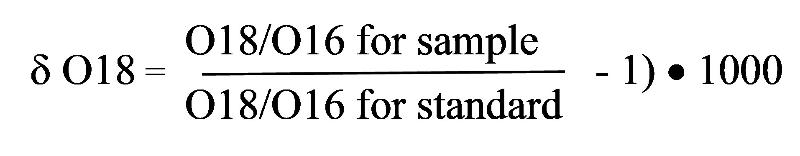
The standard usually chosen for oxygen is Standard Mean Ocean Water (SMOW). Notice the negative one in the expression. This will mean that a delta value of 0 will correspond to a sample that is identical to the standard (for example, we might expect a value of 0 if we took a sample of ocean water). A value greater than 0 means that the sample is enriched in O18 relative to the standard. A value less than 0 means that the sample is depleted in O18 relative to the standard,
below is a chart showing (sketched and approximate) data for the change in climate over the past 60 million years. Study this chart, paying attention to the axes and thinking about what the chart is saying.
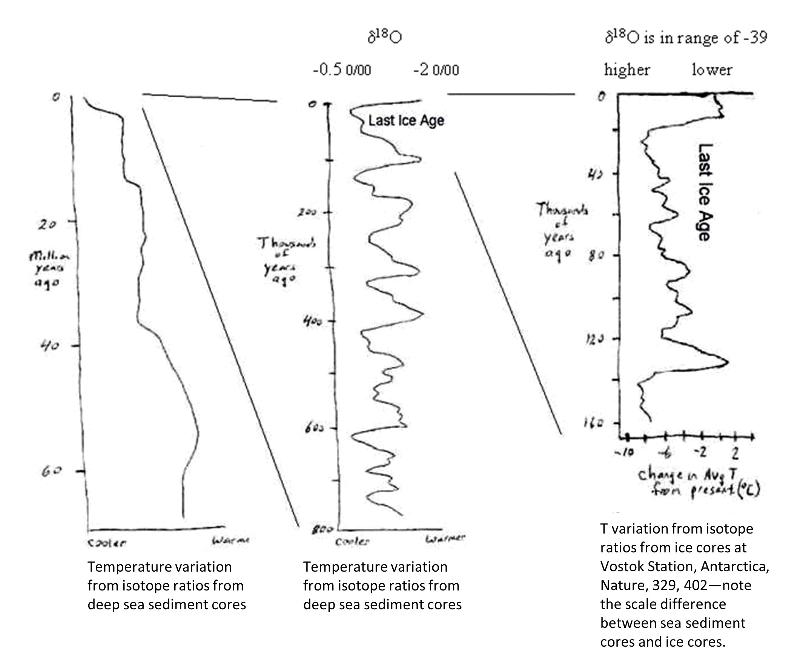
Before we dive into thinking about how isotopes have revealed Earth's past climate, let's make sure that you understand how to read this chart.
Past Climatic changes on Earth are inferred in part on the basis of changes in oxygen isotopes in ocean sediments through time. For example, researchers might take a core sample from sediments at the bottom of the ocean. Deeper sediments formed longer ago, providing a way to gather information about past ocean conditions. Thus, fossils in the sediments were formed from sea water at different periods of time and record the oxygen isotopes at the time the creature lived. Consdier the exchange reaction below, showing the exchange of O16 and O18 between sea water and carbonate ions in the calcite shell of an organism.
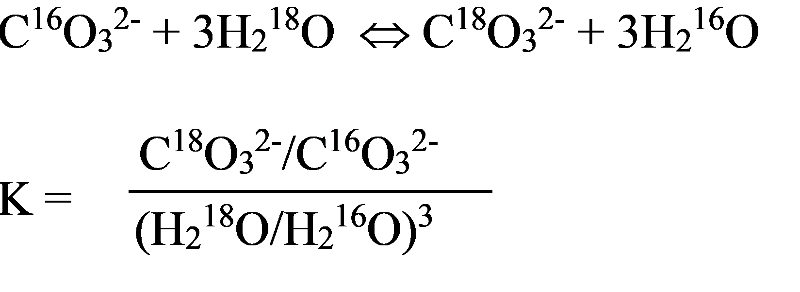
This reaction will depend on a number of factors, including water temperature and the metaboloic process of the organism, but a major factor in the concentration of O16 or O18 in the fossil will be the concentration in the sea water. More O18 in the sea water means more will be in the fossil that formed during that time.
A concept map is an effort to break one big, complex model into a serires of smaller, bite-sized conceptual steps. The following questions lead you through a concept map for the model for how climate influences the concentration of O18 in fossils deposited in the sediment at the bottom of the sea.
The partitioniing of isotopes is a function of temperature and therefore isotopes can also be used as a geothermometer to infer the temperature at which ancient ore deposits formed. Below is an example of sulfur isotope fractionation between sulfide and sulfate. Two isotopes of sulfur are 32S and 34S. The problem below is simplified so that you can get to the concept without getting lost in the complexity--for example, before using this method as a geothermometer in a real situation, you would need to also account for the effects of other variables such as pH, and to consider the fractionation involved in the precipitation of the sulfate ions as particular minerals.
H2 34S + 32SO4 2- ↔ H2 32S + 34SO4 2-
where H2S is present as a gas, and SO4 2- is present in aqueous solution (this reaction occurs in most sulfide-ore-forming situations and was also important in the formation of Carlsbad Caverns where
H2S from deep oil deposits interacted with oxygen near the water table to form sulfuric acid that formed the caverns and deposited sulfates such as gypsum.)
The equilibrium constant for this reaction is given below.
K = ([H2 32S][ 34SO4 2-])/([ H2 34S][ 32SO4 2-])
brackets indicate chemical activities, which are related closely to concentrations.
An equilibrium constant is a quantitative way of expressing the Le Chatelier principle which we examined in a previous lesson. For example, considering the Le Chatelier Principle we discussed before, increasing H2 34S on the left side of the reaction would drive the reaction toward the right, thus increasing H2 32S + 34SO4 2. If you consider the equilibrium constant, you can see that if H2 34S or 32SO42- were increased, then the values of H2 32S + 34SO4 2- would need to increase in equal measure to keep the value "K" constant. Thus, the expression for K provides a numerical measure for the Le Chaterlier Principle.
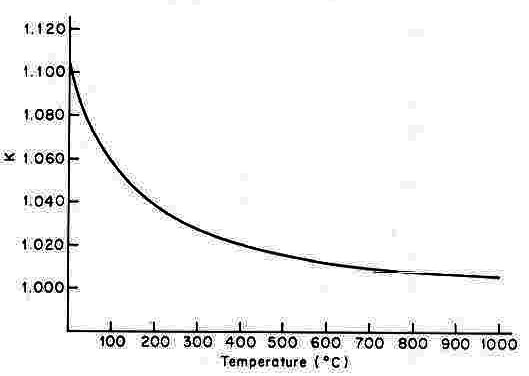
The equilibrium constant (K) for this reaction is a measure of the degree to which the two isotopes of S are fractionated between sulfide and sulfate. For example, if K=1, then there is no fractionation--that is, the proportions of isotopes are the same in sulfide and sulfate. If K = 1.1, then there is about a 10% fractionation with 34S going more into the sulfate and 32S into the sulfide. If K = 0.9, then there is about a 10% fractionation with 34S going more into the sulfide and 32S into the sulfate.
Although contstant at any particular temperature and pressure, the value of K is a function of temperature, as shown in the figure below from Stanton 1972, Ore Petrology, (an undergraduate textbook), pg 177.
last updated 5/30/2025. Text and pictures are the property of Russ Colson.